Clinical Research (CR)
Questionnaire development for QOL, patient satisfaction etc.
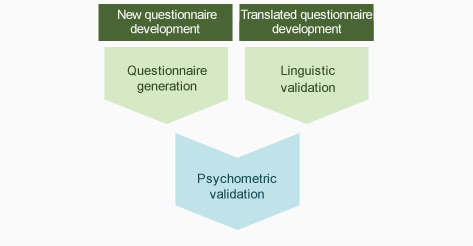
New questionnaire development
- Design of questionnaire development plan
- Consultation on development designs
- Extraction of question items and concept organization
- Question item review and documentation
- Discussion and creation of response options and scoring
- Cognitive debriefing
- interviews
- discussions with experts
- finalizing questionnaires
- writing cognitive debriefing result reports
- Reporting
- medical writing (manuscript writing assistance)
- submission support
- Point-by-point response letter writing and manuscript revision support
- support for conference slide creation
- support for conference poster creation
Translated questionnaires development
- Design of development plan
- Consultation on questionnaire development designs
- Copyright management after questionnaire development
- Forward translation
- producing drafted translation by two independent translators
- discussing drafted translation with experts
- finalizing forward translation
- Back translation
- producing back translation
- comparing back translation to original (checking for conceptual equivalence)
- discussing drafted translation with experts
- Cognitive debriefing
- interviewing
- discussing interview results with experts
- finalizing translated version
- writing cognitive debriefing result reports
- Reporting
- medical writing (manuscript writing assistance)
- submission support
- Point-by-point response letter writing and manuscript revision support
- support for conference slide creation
- support for conference poster creation
Psychometric validation (validity, reliability, and responsiveness assessments)
Proposal & plan
- Design of study plan
- Study design consultation
- Support for protocol development
- Preparation support for informed consent documents
- Application support for ethics committees
Study implementation
- Study office
- creating study-related documents
- support for participating healthcare institutions
- forming implementation teams
- arrangement of site initiation meetings
- responding to inquiries
- patient enrollment
- study progress management
- support for web survey operation
Data management
- Data management (DM)
- design of case report forms (CRFs)
- creating DM plans (DMPs)
- data input
- coding
- manual check & logical check
- issuing queries
- data lock
- writing DM reports (DMRs)
Data analysis
- Writing statistical analysis plans (SAPs)
- Statistical programming
- Statistical analysis
- Creating statistical analysis reports (SARs)
- Consultation
Reporting
- Medical writing (manuscript writing assistance)
- Submission support
- Point-by-point response letter writing and manuscript revision support
- Support for conference slide creation
- Support for conference poster creation
- Consultation