Site Management Organization (SMO)
Business Contents
Our SMO plans and organizes high-quality, accurate, cost-effective clinical trials, so that you can conduct a trial immediately. Our Clinical Research Coordinators (CRCs) are excellent at comprehending our clients’ overall clinical assessment procedures, and designing, planning, and implementing your studies. They excel at collecting and analyzing data, and at reporting your findings. We believe that it is essential for CRCs to understand where they are in the course of the study, as this is a cornerstone of achieving high-quality.
Services
Our SMO provides healthcare institutions and networks with the following support:
- Coordinating and managing clinical trials by CRCs
- Establishing and managing a central trial office
- Establishing and managing Internal Review Boards (IRBs) and ethics committees
- Developing implementation systems for trials [developing Standard Operating Procedures (SOP)]
- Managing quality of trial data
* English-speaking staff are available. Proofreading services are also available if needed.
Experience
As of Sep. 30, 2017
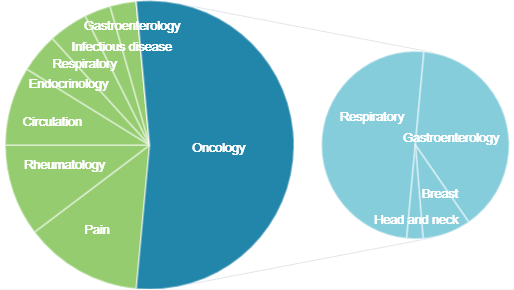
Contracts by area
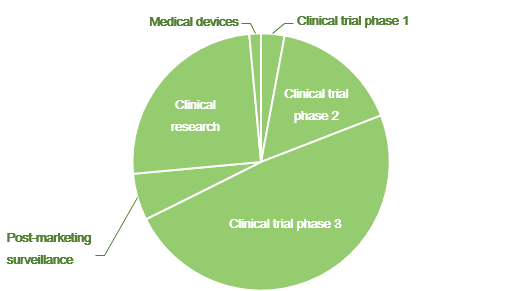
Contracts by phase
Number of protocols contracted by area and phase
| Area | Total | Clinical trial phase 1 | Clinical trial phase 2 | Clinical trial phase 3 | Post-marketing surveillance | Clinical research | Medical devices |
|---|---|---|---|---|---|---|---|
| Oncology | 36 | 2 | 10 | 16 | 0 | 7 | 1 |
| Pain | 9 | 0 | 1 | 7 | 0 | 1 | 0 |
| Rheumatology | 7 | 0 | 0 | 2 | 4 | 1 | 0 |
| Circulation | 6 | 0 | 0 | 1 | 0 | 5 | 0 |
| Endocrinology | 3 | 0 | 0 | 0 | 0 | 3 | 0 |
| Respiratory | 3 | 0 | 0 | 3 | 0 | 0 | 0 |
| Infectious disease | 2 | 0 | 0 | 2 | 0 | 0 | 0 |
| Gastroenterology | 2 | 0 | 0 | 2 | 0 | 0 | 0 |
*Number of good clinical practice (GCP) inspections undergone and results:
- 1 in 2008 – passed, 1 in 2010 – passed, 1 in 2016 – passed